STRUCTURE OF THE ATOM
 |
| google image |
1a) Charged particles in
matter :-
- Atoms have three types of sub atomic particles. They are electrons, protons and neutrons.
- Electrons are negatively charged (e-), protons are positively charged (p+) and neutrons have no charge (n).
- The mass of an electron is 1/2000 the mass of a hydrogen atom.
- The mass of a proton is equal to the mass of a hydrogen atom and is taken as 1 unit.
- The mass of a neutron is equal to the mass of a hydrogen atom and is and is taken as 1 unit.
b) Discovery of sub
atomic particles :-
In 1900, J.J.Thomson discovered the presence of the
negatively charged particles called electrons in the atom.
In 1886, E. Goldstein discovered new radiations in gas discharge and
called them canal rays. These rays were positively charged. This later led to
the discovery of the positively charged particles called protons in the atom.
In 1932 Chadwick discovered the presence of particles having no charge
in the atom called neutrons.
2) Structure of an atom
:-
a) Thomson’s model of an atom :-
(PLUM PUDDING or RAISIN BUN MODEL)
According to Thomson an
atom is similar to a Christmas pudding. The pudding had positive charge and the
electrons having negative charge were like plums on the pudding.
He proposed that :-
i) An atom consists of a positively charged
sphere and the electrons are
embedded in it.
ii)
The negative and positive charges are equal in magnitude So the atom as a whole
is electrically neutral.
b) Rutherford’s model of an atom :-
Rutherford’s
alpha scattering experiment :-
Rutherford allowed a beam of fast moving
alpha particles ( α –
particles)
having positive charge to fall on a thin gold foil. He observed that :-
i) Most of the α – particles
passed straight through the gold foil.
ii) Some of the α – particles
were slightly deflected by small angles.
iii) Very few α – particles
appeared to rebound.
Conclusions
from Rutherford’s alpha scattering
experiment :-
experiment :-
i) Most of the space inside an atom is
empty. (because most of the α –
particles passed straight through the
gold foil.)
ii) The atom had a small nucleus having
positive charge. ( because
some of the α –
particles having positive charge were
slightly
deflected by small angles.)
iii)
The size of the nucleus is very small compared to the size of the
atom. (because very few α – particles
appeared to rebound and
most of the positive charge and mass of
the atom is in the nucleus.)
Rutherford’s model of an atom :-
i) An atom has a positively
charged nucleus at its centre and most of
the mass of the atom is in the nucleus.
ii) The electrons revolve around the nucleus
in different orbits.
iii)
The size of the nucleus is very small compared to the size of the
atom.
Defects of Rutherford’s model of the atom
:-
Any particle in a circular
orbit would undergo acceleration and during acceleration the charged particle
would radiate energy. So the revolving electrons would lose energy and fall
into the nucleus and the atom would be unstable. We know that atoms are stable.
 |
| GOOGLE IMAGE |
c) Bohr’s model of an atom :-
i) An atom has a positively charged nucleus
at its centre and most of
the mass of the atom is in the nucleus.
ii)
The electrons revolve around the nucleus in special orbits called
discrete orbits.
iii)
These orbits are called shells or energy levels and are represented
by the letters K, L, M, N etc. or
numbered as 1, 2, 3, 4, etc.
iv)
While revolving in the discrete orbits the electrons do not radiate
energy.
 |
| google image |
 |
| google image |
3) Distribution of electrons in different shells :-
The distribution of electrons in the different shells was suggested by Bohr and Bury. The following are the rules for filling electrons in the
different shells.
i)
The maximum number of electrons in a shell is given by
the formula 2n2 where n is the number of the
shell 1, 2, 3
etc.
First shell or K shell can have
= 2n2 = 2 x 12 = 2x1x1 = 2 electrons
Second shell or L shell can have = 2n2 = 2 x 22 = 2x2x2 = 8 electrons
Third shell or M shell can have = 2n2 = 2 x 32 = 2x3x3 = 18 electrons
Fourth shell or N shell can have
= 2n2 = 2 x 42 = 2x4x4 = 32 electrons
and so on.
ii) The maximum number of electrons that can be filled
in the
outermost shell is 8.
iii) Electrons cannot be filled in a shell unless the
inner shells are
filled.
Composition of the atoms of the first eighteen elements :-
4) Valency
:-
- Valency is the combining capacity of an atom of an element.
- The electrons present in the outermost shell of an atom are called valence electrons.
- If an atom’s outermost shell is completely filled, they are inert or least reactive and their combining capacity or valency is zero.
- Of the inert elements Helium atom has 2 electrons in the outermost shell and the atoms of other elements have 8 electrons in their outermost shell. Atoms having 8 electrons in their outermost shell is having octet configuration and are stable.
- If an atom’s outermost shell is not completely filled it is not stable. It will try to attain stability by losing, gaining or sharing electrons with other atoms to attain octet configuration.
- The number of electrons lost, gained or shared by an atom to attain octet configuration is the combining capacity or valency of the element
Eg :- Hydrogen, Lithium, Sodium atoms can
easily lose 1 electron and become stable. So their valency is 1. Magnesium can
easily lose 2 electrons. So its valency is 2. Aluminium can easily lose 3
electrons. So its valency is 3. Carbon shares 4 electrons. So its valency is 4. Fluorine can easily gain 1
electron and become stable. So its valency is 1. Oxygen can easily gain 2
electrons. So its valency is 2. Nitrogen can easily gain 3 electrons. So its
valency is 3.
5) Atomic number and Mass number :-
a)
Atomic number (Z) :-
The atomic number of an element is the
number of protons present in the
nucleus
of the atom of the element.
All the atoms of an element have the same
atomic number.
Eg :- Hydrogen – Atomic number = 1 (1
proton)
Helium - Atomic number = 2 (2 protons)
Lithium - Atomic number = 3 (3 protons)
b)
Mass number (A) :-
The mass number of an element is the sum of the number of protons and
neutrons
(nucleons) present in the nucleus of an atom of the element.
The mass of an atom is mainly the mass of
the protons and neutrons in the nucleus of the atom.
Eg
:- Carbon – Mass number = 12 (6 protons + 6 neutrons) Mass = 12u
Aluminium – Mass number = 27 (13 protons +
14 neutrons) Mass = 27u
Sulphur – Mass number = 32 (16 protons
+ 16 neutrons) Mass = 32u
In the notation of an atom the atomic
number and mass number are written as:-
 |
| google image |
 |
| google image |
6)
Isotopes :-
Isotopes are atoms of the same element
having the same atomic numbers but different mass numbers.
Eg :- Hydrogen has three isotopes. They are
Protium, Deuterium (D) and Tritium (T).
Carbon has two common isotopes C-12 and C-14 (Though a less common but stable C-13 is also present). They are :-
 |
| google image |
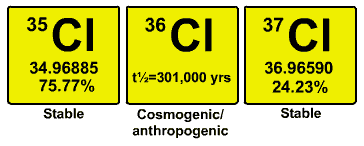
Chlorine has two main isotopes Cl-35 & Cl-37 (though a less common Cl-36 is also formed).They are :-
 |
| google image |
7)
Isobars :-
Isobars are atoms of different elements
having different atomic numbers but same mass numbers.
These pairs of elements have the same
number of nucleons.
Eg :- Calcium (Ca) – atomic number - 20 and
Argon (Ar) – atomic number 18 have different atomic numbers but have the same
mass numbers – 40.
Iron (Fe) and Nickel (Ni) have
different atomic numbers but have the same atomic mass numbers – 58.













No comments:
Post a Comment